Identifying the molecular mechanism of action of an investigational ocular drug
Zach Zixuan Shao, Dan Zhou
In collaboration with a local pharmaceutical company, we are currently investigating a therapeutic oligopeptide that is potent in treating several blinding eye diseases (eg. age-related macular degeneration and diabetic retinopathy). Clinical studies using the drug showed significant vision improvement, yet its molecular mechanism of action remains unknown. Our research focuses on identifying its locus of binding and the downstream molecular pathways responsible for the therapeutic effects. We are currently employing ligand capture and cell labeling methods to identify the binding partners as well as bioinformatic tools to discover the affected cellular pathways. Results from our studies will shed light on the pathophysiology of the relevant retinal diseases as well as better treatment of these and other related complications.

OCT Scan of the back of the eye showing drug treatment reduced inflammatory edema and restored vision
Seeing clearly: Using protein nanofibers to promote orderly corneal wound healing
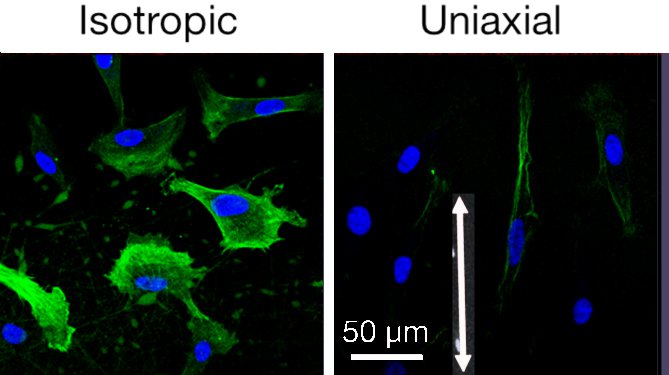
Amy Fu
The study evaluates the ability of electrospun protein nanofibers to display topographical and biochemical cues to support epithelial closure, foster fibroblast recruitment and mitigate myofibroblast phenotype. Gelatin electrospun nanofibers were used to present integrin binding sites and mechanical cues. By control of the electrode geometry, we prepare nanofiber mats with three types of orientation: isotropic, radial, and uniaxial. Rates of epithelial and fibroblast cell migration in vitro, measured using a mock wound healing assay, showed a result for epithelial cells. While fibroblasts (and a variety of cells in the literature) migrate rapidly on oriented nanofibers, corneal epithelial cells migrate faster on isotropic nanofibers than on aligned nanofibers or planar controls. Of particular importance, production of smooth muscle actin (αSMA, green in image) by myofibroblasts (TGF-β transformed fibroblasts) was reduced in cells cultured on oriented nanofiber substrates. Nanofiber diameter over the range 100–220 nm does not measurably affect migration or αSMA expression. The molecular mechanism by which myofibroblasts respond to topographical cues was explored using siRNA transfected cells with knockdown of kinases associated with integrin mediated response (FAK and Raf-1) and transcriptional regulators associated with mechanotransduction (YAP and TAZ). We were surprised that the response does not involve integrin-mediated transduction; it involves YAP and TAZ (indicative of mechanostranduction). Preliminary in vivo experiments in mice and rabbits showed reepithelialization occurs as quickly over nanofiber scaffolds as it does over the native corneal stroma. The population of inflammatory cells was reduced in wound beds treated with nanofiber scaffolds relative to untreated controls. Oriented nanofiber scaffolds do not elicit an inflammatory response and have an anti-inflammatory effect. Oriented nanofiber substrates are well suited for corneal wound applications due to their transparency, non-cytotoxicity and ability to modulate the myofibroblast phenotype.